DermaSensor Optical Spectroscopy Technology
Safety and Effectiveness Results Across Multiple Clinical Studies:
- DERM-ASSESS II Prospective Skin Cancer Validation Study1
- DERM-ASSESS II Prospective Clinical Utility Study2
- DERM-ASSESS III Prospective Melanoma Validation Study3
- PATIENT-SELECT Prospective Specificity Validation Study9
DermaSensor and Dermatologist Sensitivity for Melanoma and All Skin Cancers3
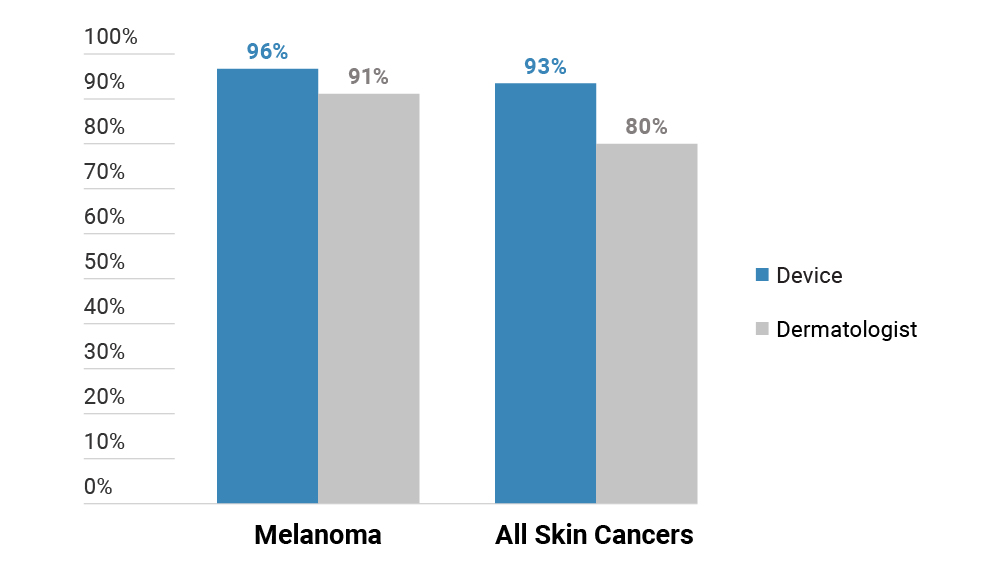
DermaSensor and GP Sensitivity for Skin Cancer2
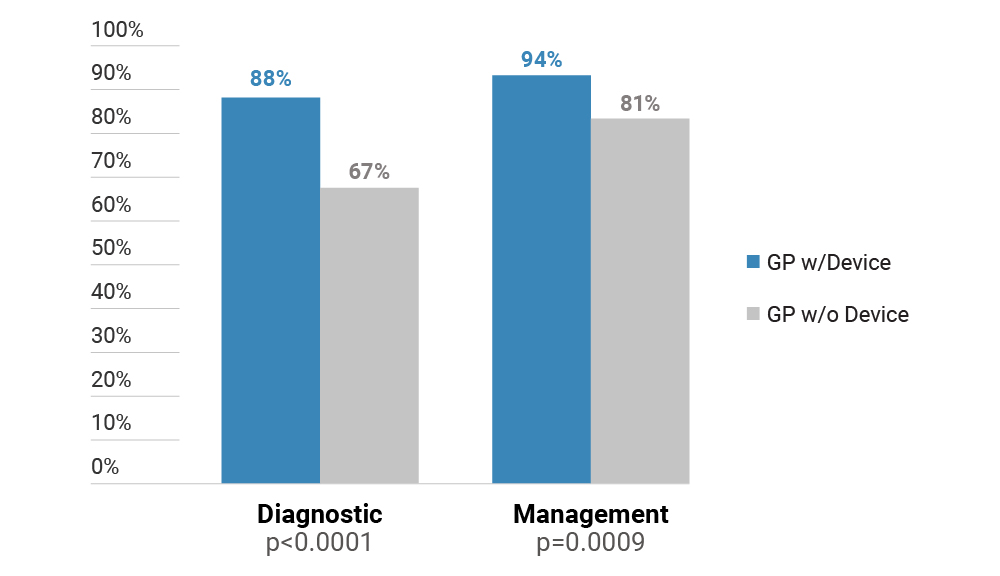
Overall study performance summary:
All clinical validation studies reported no adverse events, and studies showed DermaSensor sensitivity superior to GPs and similar to Dermatologists.1,3,4
DermaSensor’s sensitivity for melanoma ranged from 88-97% based on dermatopathology.1,3,4,10
For non-melanoma skin cancer (NMSC), i.e. BCC and SCC, DermaSensor’s performance was 88-98% based on dermatopathology.1,3,4
- There were no adverse events among over 2,000 enrolled lesions from DERM-ASSESS II, DERM-ASSESS III, and DERM-SUCCESS.1,3,4
- In DERM-ASSESS II, there was no statistical difference between the sensitivity of DermaSensor (97%) and dermatologists’ (96%) across skin cancer types.1
- In a blinded, prospective study with 10 dermatology study centers in the U.S. and Australia, DermaSensor was found to have melanoma sensitivity comparable to the dermatologists’ in diagnosing melanoma (DermaSensor: 96%; Investigators: 91%).3
- Across 1,500+ lesions in a blinded, prospective study with 22 GP study sites in the U.S. and Australia, DermaSensor’s sensitivity results for melanoma, SCC and BCC were 88%, 98%, and 98%, respectively.4
- Specificity of the device ranged from 27-67% for benign nevi, 19-70% for SKs, and 7-57% for AKs.1,3,4,9
- An Independent Investigator-Initiated Study conducted in NZ found a 98% sensitivity of the device across all skin cancers.5
- DermaSensor's specificity was 61% for lesions of concern to patients, 36-37% for unbiopsied lesions of concern for non-specialist HCPs, and 21-33% for physician biopsied lesions.1,4,7,8,9
- As reported in Nature in 2019, "... most skin lesions are diagnosed by primary care doctors, and problems with inaccuracy have been underscored; if AI can be reliably shown to simulate experienced dermatologists, that would represent a significant advance."6
- In a randomized, prospective study of DermaSensor utility with 57 GPs, these physicians made over 5,000 assessments of skin lesions. DermaSensor increased physicians’ cancer detection sensitivity from 81% to 94%, and this improvement was statistically significant (p = .0009).2 There was no statistically significant change in the GP’s specificity, or false positive rate, for benign lesions (p = .3558).2
DERM-ASSESS II Summary Table2,7,8
| DERM-ASSESS II | Device | Dermatologist |
|---|---|---|
| All skin cancers | 97% | 96% |
| Melanoma | 100%* | 90% |
| SCC | 97% | 96% |
| BCC | 97% | 100% |
*Note: There were only 20 melanomas included in the sample and the study was not powered for melanoma detection. |
||
DERM-ASSESS III Summary Table3
| DERM-ASSESS III | Device | Dermatologist |
|---|---|---|
| All skin cancers | 93% | 80% |
| Melanoma | 96% | 91% |
| Melanoma and Highly Atypical Nevi | 91% | 72% |
| PATIENT-SELECT | Device | GP Assessment |
|---|---|---|
| All skin cancers | 90% | 40% |
Note: Device and PCPs performance assessed on biopsy diagnosis when available, and dermatologist-panel diagnosis when pathology was unavailable. |
||
DermaSensor™ Number Needed to Biopsy, NPV⁄PPV Performance Across Spectral Score Ranges 1-10
DermaSensor Number Needed To Biopsy & PPV
| Lesion Type and Study | NPV | PPV | Device NNB |
|---|---|---|---|
| GPs for All Skin Cancers | 97%4 | 17%4 | 6.74 |
| Specialists for Melanoma | 98%3 | 16%3 | 6.33 |
| Specialists for All High Risk Lesions | 96%3 | 23%3 | 4.43 |
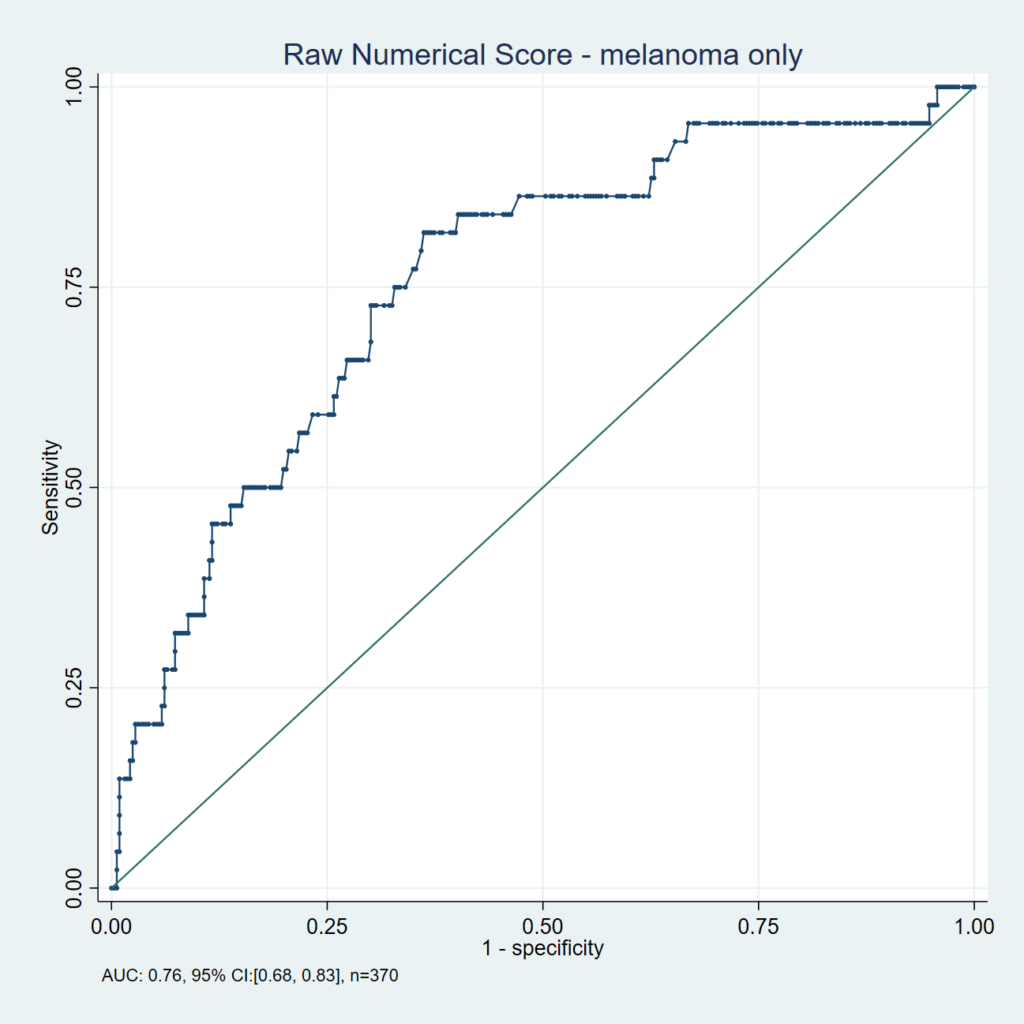
ROC Curve for the DermaSensor Device for Melanoma
The Receiver Operating Characteristic (ROC) curve for the DermaSensor device demonstrates the diagnostic capabilities (sensitivity and specificity) of the device based on a model taking into account differences between users. The associated area under the curve (AUC) is a calculated measure of diagnostic capabilities and can be used to assess potential for improvement in detection capabilities with use of a diagnostic tool. The AUC of the DermaSensor device for melanoma was 0.76 compared to 0.75 for dermatologists.
Spectral Score Groupings3
| Spectral Scores Groupings | Melanoma PPV (NNB) | Frequency of ‘Investigate Further’ Lesions |
|---|---|---|
| 1-5 | 13% (8) | 84% |
| 6-10 | 32% (3) | 16% |
| 1-3 | 6% (17) | 46% |
| 4-7 | 18% (6) | 35% |
| 8-10 | 40% (2) | 19% |
Note: Number neeeded to Biopsy (NNB) assumes all positive device results were biopsied by the HCP. It is calculated by dividing 100 by the PPV. |
||
DISCLAIMER: The DermaSensor device is currently TGA listed and available for sale in Australia. In the U.S., it is for investigational use and is not currently cleared or available for sale.
1Manolakos D, Rabinovitz H, Geisse J, Bonning M, Rodriguez-Diaz E, Bigio I, Cognetta A. Clinical Validation of a Handheld Elastic Scattering Spectroscopy-Artificial Intelligence Device. Presentation, AAD Innovations Academy, July 21-24, 2022.
2Tepedino K, Tablada A, Barnes E, Da Silva, T. Clinical Utility of a Handheld Elastic Scattering Spectroscopy Tool and Machine Learning on the Diagnosis and Management of Skin Cancer by Primary Care Physicians. Poster Presentation, SDPA Fall Conference, Nov 4-7, 2021.
3Hartman, R. Tepedino, K., Fung, MA., McNiff, JM., Grant-Kels, J. Clinical Validation of a Handheld Elastic Scattering Spectroscopic Device in the Evaluation of Lesions Suggestive of Melanoma, Presentation at the American Academy of Dermatologists Annual Meeting, Mar 24-28th, 2022.
4Data on file, DermaSensor, Inc.
5Salmon P and Bonning M. Use of Elastic-scattering Spectroscopy and Machine Learning When Assessing Skin Lesions Suggestive of Skin Cancer, Poster Presentation, SDPA Fall Conference, Nov 4-7, 2021.
6Topol E. High-performance medicine: the convergence of human and artificial intelligence. Nature Medicine. 2019;25:44-66.
7For lesions biopsied in DERM- ASSESS II, dermatologist performance (i.e. dermatologist sensitivity and specificity) is based on the study dermatologists’ in-person binary assessment of biopsied lesions as being malignant or benign, prior to receiving pathology results.
8For unbiopsied lesions the dermatologists’ clinical determination of the lesion as benign was used as the reference standard; however, for the dermatologists’ clinical assessment there was no reference standard since no biopsies were performed and accordingly no specificity is reported for their evaluations.
9Tepedino M, Balthazar D, Hucks C, Zeitouni N. Use of Elastic Scattering Spectroscopy on Patient-Selected Lesions That are Concerning for Skin Cancer, Poster Presentation, American Dermoscopy Meeting, Jun 29-Jul 2, 2022.
10Includes melanoma plus severely atypical nevi.
Learn more about our groundbreaking device.
80-0006.4 v2